Something strange was hiding in the Horsehead. The nebula, named for its stallionlike silhouette, is a towering cloud of dust and gas 1,500 light-years from Earth where new stars are continually born. It is one of the most recognizable celestial objects, and scientists have studied it intensely. But in 2011 astronomers at the Institute of Millimeter Radioastronomy (IRAM) and elsewhere probed it again. With IRAM's 30-meter telescope in the Spanish Sierra Nevada, they homed in on two portions of the horse's mane in radio light. They were not interested in taking more pictures of the Horsehead; instead they were after spectra—readings of the light broken down into their constituent wavelengths, which reveal the chemical makeup of the nebula. Displayed on screen, the data looked like blips on a heart monitor; each wiggle indicated that some molecule in the nebula had emitted light of a particular wavelength.
Every molecule in the universe makes its own characteristic wiggles based on the orientation of the protons, neutrons and electrons within it. Most of the wiggles in the Horsehead data were easily attributable to common chemicals such as carbon monoxide, formaldehyde and neutral carbon. But one spot in the Horsehead also had several small unidentified lines equally separated from one another in frequency. These represented a mystery—a molecule completely unknown to science.
Immediately after seeing the data, Evelyne Roueff of Paris Observatory and other chemists on the team started hypothesizing about what kind of molecule might create the signal. They concluded that the unknown species had to be a linear molecule—a compound whose atoms are arranged in a straight chain. Only a certain type of linear molecule would produce the spectral pattern the chemists were seeing. After working through lists of likely molecules, they hit on C3H+, propynylidynium. This molecular ion had never been seen before. In fact, there was no proof it existed at all. If it could form, it would be highly unstable. On Earth it would almost immediately react with something else to transform into a more settled species. But in space, where the pressure is low and molecules rarely run into anything else to bond with, C3H+ might just be able to survive.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Roueff and her colleagues studied whether the Horsehead Nebula contained the right ingredients and conditions to form the molecule. In 2012 they published a paper in Astronomy & Astrophysics concluding that the wiggle they saw likely belonged to C3H+. “I was relatively confident myself,” Roueff says. “But it required about two to three years to convince everyone that we had the right identification.”
At first, some skeptics contested the claim—if C3H+ had never been seen before, how could they be sure this was it? The clincher came in 2014, when researchers at the University of Cologne in Germany managed to create C3H+ very briefly in a laboratory. Not only did the feat prove that the molecule could exist, it also allowed scientists to measure the spectrum it produces when excited—the very same spectrum that showed up in the Horsehead. “It's very rewarding to find a new molecule that we did not really think about before,” Roueff says. “When you are able to identify it through a chain of logic, it's like being a detective.”
One alien molecule down, many, many more to go. The Horsehead Nebula is no aberration. Almost everywhere in the universe astronomers look—if they peer closely enough—they see unidentified spectral lines. The compounds we humans are familiar with, the species responsible for the huge diversity of materials on this planet, are just a fraction of those nature has created. And finally, after decades of work developing theoretical models and computer simulation techniques, along with laboratory experiments to reproduce new molecules, astrochemists are putting names to many of those unidentified lines.
Empty Space
As recently as the 1960s, most scientists doubted molecules could exist in interstellar space at all—the radiation there was thought to be too harsh for anything much beyond atoms and a few basic free radicals to survive. In 1968 physicist Charles Townes of the University of California, Berkeley, decided to look for molecules in space anyway. “I got the feeling that most of the Berkeley astronomers thought my idea was a little wild,” Townes, a Nobel laureate who died in 2015, recalled in a 2006 account for the Astronomical Society of the Pacific. But Townes forged ahead and built a new amplifier for the six-meter antenna at Hat Creek Radio Observatory in California, which revealed the presence of ammonia in the Sagittarius B2 cloud. “How easy, and how exciting!” he wrote. “The news media as well as scientists began buzzing us.”
In the years since, astronomers have found more than 200 types of molecules floating in space. Many are quite different from the species seen on the ground. “We usually do chemistry based on the conditions we have on Earth,” says Ryan Fortenberry, an astrochemist at Georgia Southern University. “When we get away from that paradigm, the chemicals that can be created are unbounded. If you can dream of a molecule, no matter how bizarre, there is a finite probability that over the eons of time and the immensity of space it has existed somewhere.”
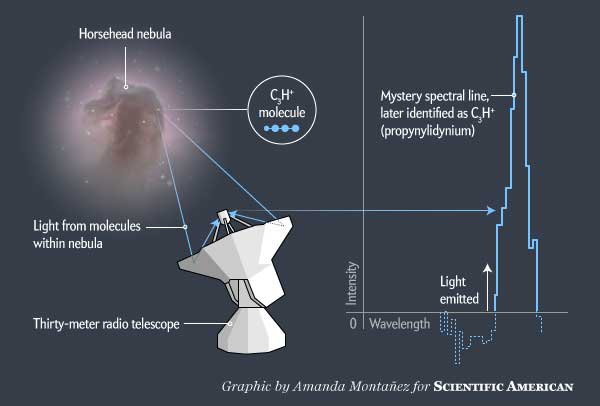
Astronomers observing the Horsehead nebula with a radio telescope in Spain saw the chemical signature of a mystery molecule. The telescope returned spectroscopic data—a line graph representing the intensity of light coming from the nebula across a range of wavelengths. Sharp rises in the light, such as the feature shown here, indicate the presence of a specific molecule whose chemical properties allow it to emit that particular wavelength of light. After much sleuthing researchers were able to determine that this unidentified line feature comes from the never-before-seen compound C3H+, which exists only in space.
Space is literally an alien environment. Temperatures can be much, much hotter than on Earth (such as in the atmosphere of a star) and much, much colder (in relatively empty interstellar space). Likewise, the pressures (extremely high or low) are far from terrestrial. Consequently, molecules can form in space that would never emerge on our planet—and then they can stick around, even if they are highly reactive. “A molecule can go years and years before it bounces into another molecule in interstellar space,” says Timothy Lee, an astrophysicist at NASA's Ames Research Center. “It might be in a region where there's no radiation, so even if it's not that stable, it can exist for a long time.”
These space molecules, once identified, could teach us a lot. Some of them might prove beneficial if scientists can create them in labs and learn to exploit their properties. Other molecules may help explain the origins of the organic compounds that gave rise to life on Earth. And all of them stand to expand the bounds of what is possible for chemistry in the universe.
Game-Changing Telescopes
In the past decade, as powerful new telescopes capable of observing faint spectral lines have come online, the search for alien molecules has accelerated. “It's a heyday for astrochemistry right now,” says Susanna Widicus Weaver, who leads an astrochemistry group at Emory University. The data available even in 2015, she says, are a huge improvement from just a decade earlier when she finished her doctorate. NASA's high-altitude Stratospheric Observatory for Infrared Astronomy (SOFIA), mounted on the side of a Boeing 747SP, began observing in infrared and microwave light in 2010 and the European Space Agency's Herschel Space Observatory launched into orbit in 2009 to target the same wavelengths.

In 1968 astronomers discovered ammonia in the Sagittarius B2 Cloud. Credit: ESO/APEX and MSX/IPAC/NASA
The real game changer, however, is the multinational Atacama Large Millimeter/submillimeter Array (ALMA), a constellation of 66 radio dishes inaugurated in 2013. At an altitude of about 5,200 meters on the Chajnantor Plateau, a Mars-like red expanse in Chile's Atacama Desert, the driest place in the world, ALMA's matching antennas swivel in unison as observers collect light from cosmic objects. Extremely dark and clear skies nearly devoid of image-blurring moisture give the telescope unprecedented sensitivity and precision in wavelengths from infrared to radio. ALMA creates both a visual picture and a spectrum for every pixel of its images, producing tens of thousands of spectral lines in every field of view it observes. “It's amazing and it's overwhelming at the same time,” Widicus Weaver says. “These data sets are so big that they often have to mail them to scientists on flash drives because they can't download them.” The flood of data is providing a wealth of new spectral lines for astrochemists to mine. But like unidentified fingerprints at a crime scene, these lines are useless to scientists unless they can determine which molecules created them.
Finding a Link
To match molecules to these lines, scientists can go in a few directions. As in the case of C3H+, astrochemists might start with clues from the spectrum to guess what molecule might be behind it. A technique called ab initio quantum chemistry (ab initio is Latin for “from the beginning”) allows scientists to start from pure quantum mechanics—the theory that describes the behavior of subatomic particles—to calculate a molecule's properties based on the motions of the protons, neutrons and electrons in the atoms that compose it. On a supercomputer, scientists can run repeated simulations of a molecule, each time slightly adjusting its structure and the arrangement of its particles, and watch the results to find the optimal geometry of a compound. “With quantum chemistry we're not limited by what we can synthesize,” Fortenberry says. “We're limited by the size of the molecules. We need large amounts of computational power to do the calculations.”
Researchers can also look for hard evidence of new molecules by creating them in a lab and directly measuring their spectral features. A common technique is to start with a chamber of gas and run a current of electricity through it. An electron in the current might collide with a molecule of gas and break its chemical bond, giving rise to something new. Researchers keep the gas at very low pressure so that any chemicals that arise have a chance to hang out for a few moments before running into another molecule and reacting. Scientists will then shine various wavelengths of light through the chamber to measure the spectrum of whatever is inside. “You can get to the point where you've produced in the lab the same molecule that's occurring in space, but you don't necessarily know what the molecule is,” says Michael McCarthy, a physicist at the Harvard–Smithsonian Center for Astrophysics. “So then you have to try to infer the elemental composition from a combination of different lab experiments with different samples.”

Atacama Large Millimeter/submillimeter Array (ALMA) in Chile's Atacama Desert. Credit: NRAO
In 2006 McCarthy and his colleagues created the negatively charged molecule C6H− and measured its spectrum. Soon afterward they found the same spectral features in the interstellar Taurus Molecular Cloud around 430 light-years away. Previous searches for negative molecules in space had come up empty, so many scientists doubted whether they existed in significant numbers. “It led us to a whole set of discoveries in which we were able to detect related molecules in the lab and then in space,” McCarthy says. His team and others have now found C6H− in more than a dozen cosmic sources.
And in the 1980s scientists trying to make new chemicals produced the molecule argonium (36ArH+), a strange compound not normally found on Earth that combines hydrogen with the generally inert gas argon. In 2013 astronomers found argonium in space, first in the Crab Nebula and later in a distant galaxy via ALMA observations. Compounds based on noble gases form only under very specific circumstances; scientists think that in space, high-energy charged particles called cosmic rays slam into argon and knock electrons loose, enabling it to bond with hydrogen. For this reason, if scientists see argonium in a region of space, they can surmise that the place is flooded with cosmic rays. “It's a very specific indicator of these circumstances, which actually are very important in space,” says Holger Müller of the University of Cologne, leader of the team behind the ALMA discovery.
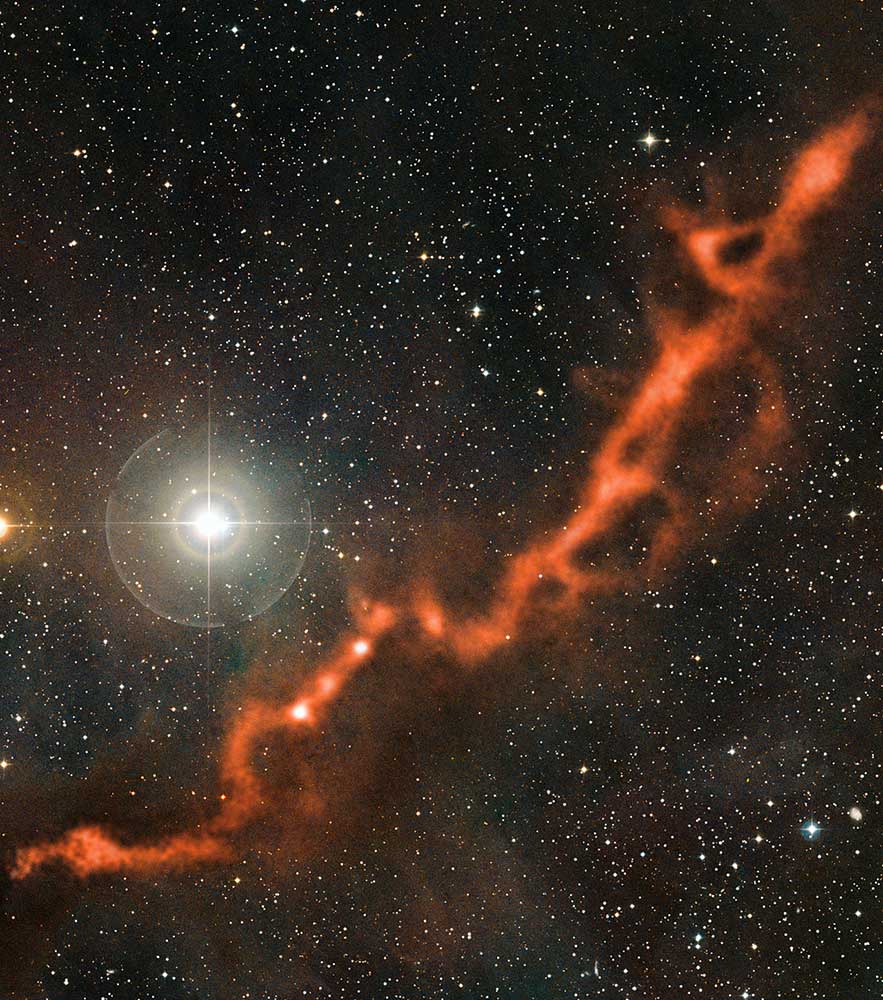
Taurus Molecular Cloud, as seen by the Atacama Path-finder Experiment (APEX) telescope in Chile. Credit: ESO/APEX (MPIfR/ESO/OSO)/A. Hacar et al./Digitized Sky Survey 2. Acknowledgment: Davide De Martin
A New World of Molecules
Many of the molecules lurking in stars and nebulae are foreign in the extreme. To ask what they would look or feel like if you could hold them in your hand is nonsensical because you could never hold them—wthey would react immediately. If you did manage to make contact with them, they would almost certainly prove toxic and carcinogenic. Oddly, however, scientists have a rough idea of what some alien molecules would smell like: many detected so far belong to a class of compounds called aromatics, which are derived from benzene (C6H6) and were originally named for their strong odors.
Some new compounds reveal surprising atomic structures and share charge between atoms in unforeseen ways. Sometimes they challenge current theories of molecular bonding. A recent example is the molecule SiCSi, discovered in 2015 in a dying star, which is made of two silicon atoms and one carbon atom that are bonded in an unexpected way. The resulting molecule is somewhat floppy and produces a spectrum different from what simple theoretical models predict.
And space molecules may help answer one of the universe's most fundamental questions: How did life get started? Scientists do not know if amino acids, the building blocks of life, first arose on Earth or in space (and were then delivered to our planet by comets and meteorites). “The big question is, Do they form in molecular clouds as a star is forming,” asks Widicus Weaver, “or do they form once you have a planet or some other chunk of rock where chemistry can occur on the surface?” The answer will determine whether it is likely that amino acids are plentiful in the universe and available to possibly seed life on the myriad exoplanets out there or whether the chemistry that sparked us was isolated to our own planetary cradle. Astrochemists have already spotted signs of amino acids in space as well as sequences of molecules that might have given rise to them.
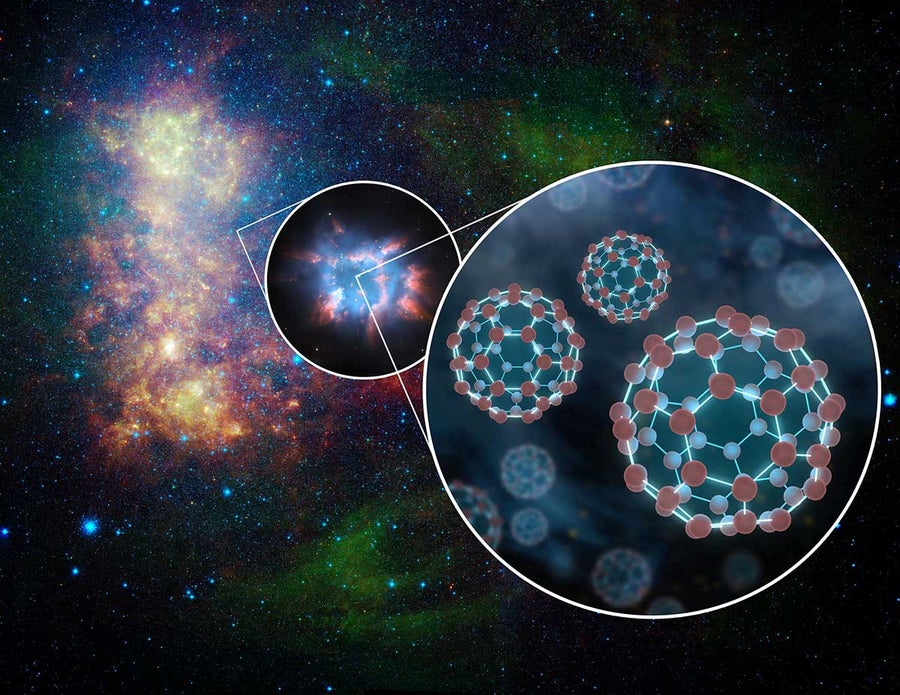
Buckyballs, conglomerations of 60 carbon atoms, were first created in labs on Earth and later discovered in space. Credit: NASA/JPL-Caltech
Finally, some rare species could prove useful if they can be created in sufficient quantities and kept under controlled conditions. “The great hope of astrochemistry is that we can find molecules with completely new properties and apply those to problems here on Earth,” Fortenberry says. An example is the soccer ball–shaped molecules “buckyballs.” These large conglomerations of 60 carbon atoms first showed up in a lab in 1985 (and won their discoverers a Nobel Prize). Almost a decade later astronomers saw spectral features in interstellar gas that looked consistent with positively charged versions of buckyballs, and the connection was confirmed in 2015 when researchers matched those features to the spectrum of buckyballs created under spacelike conditions in the lab. “This molecule is now all over the galaxy and all over the universe,” noted the late buckyball co-discoverer Harold Kroto, then a chemistry professor at Florida State University. Lately buckyballs have turned out to be not just a quirk found in space but a practical tool for nanotechnology, useful for strengthening materials, for improving solar cells and even for pharmaceuticals.
At this point astrochemists are still testing the shallow waters in the great sea of molecules out there in space. The finds they have already turned up are a reminder that our own small corner of the cosmos is just that—an insignificant, and not necessarily representative, sample of what is possible. Perhaps the species we are familiar with on Earth are in fact the exotic ones, and the buckyballs, the Horsehead Nebula, C3H+ and others still unknown are the ordinary stuff of the universe.
