The First Man to Have His Genes Edited Inside His Body
A clinical trial for zinc-finger nucleases, a potential new method of curing genetic diseases, kicks off.

Updated on November 15 at 12:37 p.m ET
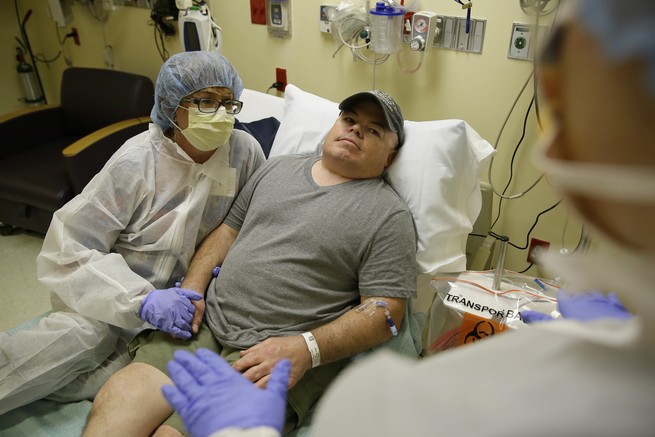
On Monday, 44-year-old Brian Madeux spent three hours hooked up to an IV and made scientific history.
The clear liquid that dripped into his arm set off a chain of events that is supposed to end with the precise insertion of a gene that Madeux has lacked since birth into the DNA of his liver cells. With that, he might be cured of Hunter syndrome, a genetic disorder that causes a range of symptoms including joint stiffness, breathing problems, and developmental delay. Madeux has had 26 surgeries to deal with it all.
If it works, the change will be permanent. If the gene gets inserted in the wrong place, that will likely be permanent too. Doctors may not know for several months.
The Associated Press broke the news of Madeux’s infusion Wednesday. He is the first patient treated in a clinical trial from Sangamo Therapeutics, which is using microscopic gene-editing tools called zinc-finger nucleases to alter DNA inside the bodies of patients. So invested is Sangamo in this technology that the company is concurrently recruiting patients with hemophilia B and Hurler syndrome for two other clinical trials using zinc-finger nucleases.
Gene therapy, in which a missing gene is inserted into a patient’s DNA, has been around since 1989. What makes Sangamo’s treatment different is precision. Past gene therapies have made use of certain viruses, which insert genes in somewhat random places in the genome—sometimes in an appropriate place, but sometimes in places that can cause cancer, which makes the treatment risky.
Sangamo’s zinc-finger nucleases are engineered to find a specific stretch of DNA, where a new gene can be safely inserted. “You know exactly where you’re going in the genome. It’s not like using a shotgun hoping you’re hitting a bird. It’s like using a rifle,” says Chester Whitley, a principal investigator on Sangamo’s clinical trial and a pediatrician at the University of Minnesota. Madeux’s infusion contained billions of copies of the gene he is missing as well as zinc-finger nucleases.
Zinc-finger nucleases are also distinct from CRISPR, an even newer gene-editing tool that can also find precise stretches of DNA. CRISPR for gene editing in the body has not reached clinical trials in the U.S. yet.
Sangamo had previously used zinc-finger nucleases to edit immune cells taken from HIV patients. Once those gene-edited cells were injected back into the patient, they were resistant to HIV infection. But now, Sangamo is inserting zinc-finger nucleases directly into the body to do their editing. It’s a new level of risk but also a new level of possible reward.
Madeux’s treatment will not be able to reverse all of the damage in his body. But it could halt the progression of the disease. It could also eliminate the need for expensive and time-consuming enzyme-replacement therapies, which many Hunter-syndrome patients currently use to manage their disorder.
But what Sangamo really hopes to do in the future is to cure kids. When I spoke to Sangamo’s president Sandy Macrae earlier this year, he told me the first trials have to be in adults because that’s the prudent thing to do. “Once we’re sure the medicine is safe and effective, our intention is to get it as quickly as possible into children,” he said.
So if it is safe and effective—still big ifs—then Madeux’s infusion could kick off a new era for genetic disorders, one where kids never have to suffer their effects in the first place.


