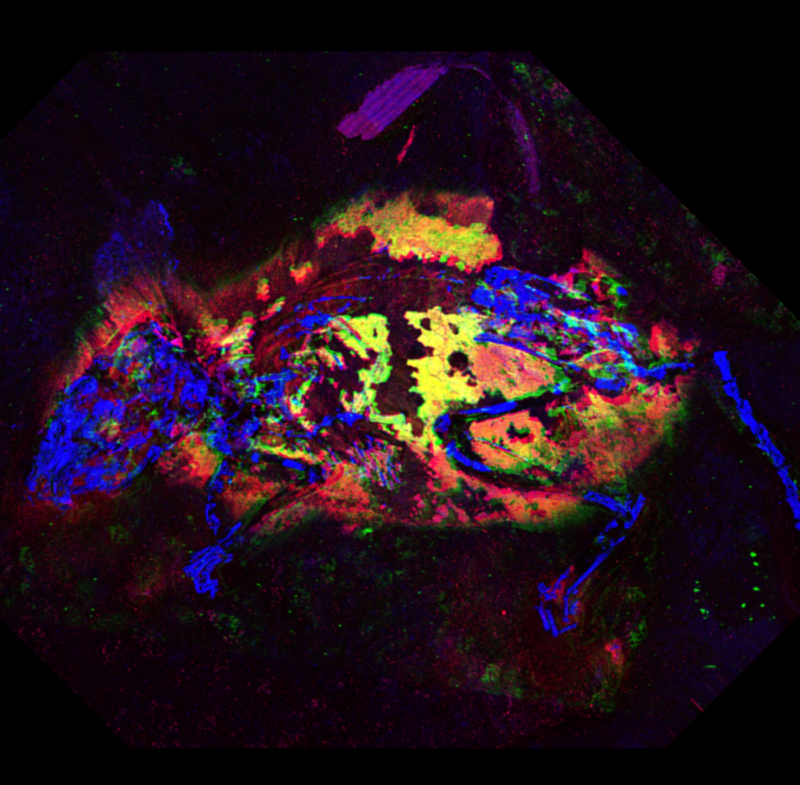
Here's something you don't hear often: the dead field mouse looks incredible for its age. It lived and died three million years ago in what is now Germany, but layers of rock preserved nearly its whole skeleton, along with most of the fur and skin on its body, feet, and tail. Even its tiny, delicate ears were preserved.
Thanks to new imaging methods and a better understanding of the chemistry behind pigment in animal fur and feathers, we now know that it had reddish-brown fur with a white underbelly. Paleontologists have had the tools to detect patterns of light and dark coloring in fossil feathers for a few years, but this is their first real glimpse of a colored pigment.
It comes in colors
The range of colors in animal fur comes from varying amounts of two types of a pigment called melanin. Eumelanin produces black or dark brown coloring, while pheomelanin creates reddish or yellow hues. Pheomelanin doesn’t tend to hold up well over the millions of years most fossils are buried; eumelanin is more sturdy, which is why we have a decent idea about the patterns of light and dark in the feathers of Archaeopteryx and some of the other ancestors of today’s birds.
In several years of studying the chemistry of pigment in modern animals, University of Manchester geochemist Roy Wogelius and his colleagues discovered that chemical compounds with rings containing sulfur are a dead giveaway for the presence of reddish pheomelanin in recently dead animal fur or feathers. But when the animal decays, those sulfur-ring molecules break down.
“We discovered an important relationship between zinc and sulfur that we believe could be used as a ‘tag’ for resolving pheomelanin in fossils,” co-author Nick Edwards, a scientist at SLAC National Accelerator Laboratory, told Ars Technica. When pheomelanin breaks down, tiny traces of the metal remain embedded in the fossilized fibers of hair, and Wogelius and his colleagues found a way to look for those traces in order to reconstruct the coloring of the long-dead fossil field mouse.
It seemed like a perfect way to test the method. “We thought that the preservation in this case was good enough to allow comparison with modern tissue, but old enough that it would be a good test of our new protocols for analyzing fossils,” Wogelius said. Using a technique called synchrotron x-ray fluorescence imaging, the team bombarded the fossil with X-rays and watched how they interacted with the metals in the fossilized strands of mouse fur. The patterns of metal traces matched the ones they had previously seen in animals with very high concentrations of the reddish pigment—everywhere except on its white underbelly.
Fossils preserve chemistry, not just shapes
“This study presents evidence that, beyond what we can see with the visible part of the electromagnetic spectrum, that original biological chemistry is also retained within these structures,” Edwards told Ars. It also shows that paleontologists may miss important information if they only check a couple of spots for the presence of pigment. Instead, say Wogelius and his colleagues, mapping how pigments vary (and often mix) across an organism’s body is the key to really understanding its coloration.
Of course, that depends on having fossilized fur or feathers to analyze in the first place. When it died 3 million years ago, the field mouse happened to fall into a small, deep basin where sediment piled up quickly, burying the mouse and sealing it away from oxygen. That kind of good luck doesn’t happen every day.
But it does happen. “Common? No. But there will be hundreds of specimens either stored in museums or yet to be sampled which will have suitable material for study,” Wogelius said. X-ray fluorescence imaging doesn’t require researchers to destroy fossil samples in order to study them, so the team is optimistic about museums’ willingness to allow a closer look at their best-preserved specimens. “We are busy with a number of new projects, some related to pigments and some related to other aspects of soft tissue preservation stretching back further than 3 million years,” said Wogelius.
“It has taken us over a decade to really understand the information that our primary technique (synchrotron X-ray fluorescence imaging) can provide,” added Edwards.
Nature Communications, 2019. DOI: 10.1038/s41467-019-10087-2;(About DOIs).
reader comments
14