Long before the coronavirus began to spread beyond China, infectious disease experts around the world knew there was ample reason to fear it. Not only was the pathogen highly contagious and lethal, it was also new—scientists had written no medical papers on it, doctors had no vaccines or pills to give their patients. The most effective tools we have, at the moment, are public health measures out of the 19th century such as quarantines and social distancing.
The emergence of Severe Acute Respiratory Syndrome Coronavirus 2019, or SARS CoV-2, has made plain our vulnerability to a novel pathogen. An estimated 160 million to 214 million people in the United States could be infected over the course of the epidemic, by some estimates. Fatalities could run from 200,000 to 1.7 million people, according to the CDC, and into the tens of millions worldwide.
The lack of treatments is a startling contrast to the sophistication of current medical science, which is in something of a golden age of genomics, machine learning and big data. The coronavirus has caught us flat-footed. Yet, at the same time, it has underscored how far the tools of medicine have evolved in recent years. Just days after local infectious disease experts sent virus samples taken from two patients infected with a suspicious form or pneumonia to the Wuhan Institute of Virology, a world-renowned research laboratory, for analysis, scientists had sequenced the newly emergent pathogen's RNA and uploaded its entire 30,000-nucleotide genetic code to the cloud.
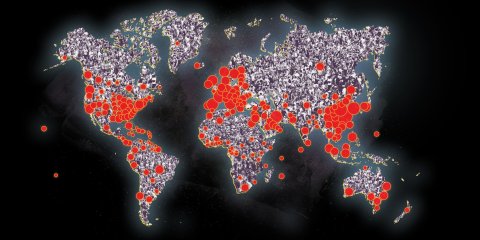
Across the globe, scientists downloaded it and began to isolate antibodies. Virologists and computational biologists used machine learning tools to analyze its structure and search for existing drugs that might work against it. Pathologists applied the tools of molecular biology to search for vulnerabilities in the virus' armor of protein. "The pace of the scientific research has been really at a breathtaking speed," says Angela Rasmussen, a virologist and research scientist at Columbia University. "It's unprecedented."
As the caseload continues to rise exponentially in the U.S. and other parts of the world, scientists are racing to find antiviral drugs that are effective in alleviating the worst ravages of the disease, a devastating pneumonia that affects an alarmingly high number of patients. The goal is to give doctors a broader range of weapons in the weeks and months ahead, and save lives.
Rapid response
In recent years, technologies that allow rapid sequencing of genetic material have become standard equipment in most top research laboratories. Because of these tools, scientists were able to state with relative confidence that the current virus is closely related to the SARS coronavirus that hit in 2003, as well as very closely related to a bat coronavirus found in a cave in Yunnan, China, back in 2017. With this knowledge, scientists dusted off the files from that outbreak and picked up where other scientists left off.
Rapid genome sequencing didn't merely allow researchers to publish the full SARS-CoV-2 sequence in days, as opposed to months in the case of the SARS genome in 2003. It also allowed scientists to sequence strains of the virus in Washington State, New York City, Italy and other parts of the world, which they are using to piece together a kind of SARS-CoV-2 ancestry registry—a detailed map of how the virus spread and mutated.
Scientists used this information to trace the progress of the virus and estimate how many people have been exposed in any given area, which informed the public health response. "We know from sequencing some of the more recent Seattle viruses, that those viruses were probably derived from the first patient who came to the U.S. with coronavirus in mid-January," says Rasmussen, who noted at the time that the Seattle area had an estimated 6,500 cases.
Tracking the virus in this way helped public health workers conclude early on that the virus was unusually contagious, which informed emergency planning in China, Italy and elsewhere. The most urgent task, of course, is to keep intensive-care wards from being overwhelmed by patients in respiratory distress. For the most critically ill patients, COVID-19 attacks the lungs, triggering the immune system to create a thick soup of white blood cells and other immune agents that flood the lungs. In the most severe cases, this immune response clogs up air cavities critical for transferring oxygen from the air to the body, greatly reducing lung capacity.
To survive, these patients require mechanical ventilators, which can force higher concentrations of oxygen into the parts of the lungs that are still functioning, allowing them to rest, recover and preserve precious energy needed to outlast the viral attack. But ventilators are in dangerously short supply. For instance, fewer than one-tenth of the 925,000 hospital beds in the U.S. are equipped for critically ill patients, who could number between 2.4 million to 21 million people in the United States, say estimates.
Antiviral medication could shorten the time patients need to be on ventilators—and perhaps prevent many of them from needing one in the first place. One of the most promising ideas is to develop new drugs that can attenuate the immune response to keep the lungs functioning adequately. Doctors in the First Affiliated Hospital of University of Science and Technology of China (Anhui Provincial Hospital) and Anhui Fuyang Second People's Hospital In China used , a drug developed by Chugai, a Japanese company, originally to treat rheumatoid arthritis, on 21 critically ill patients.
Within a few days, the fever returned to normal and all other symptoms improved "remarkably," according to a paper on the study published soon after. Fifteen of the 21 patients had lowered their oxygen intake and one patient needed no oxygen therapy. CT scans revealed that the lung function improved in 19 patients of the 21 patients, and the abnormal percentage of white blood cells found in 17 of the 21 patients before treatment returned to normal in 10 within five days. Nineteen of those treated had been released within two weeks, and the other two were reported to be "recovering well."
Regeneron Pharmaceutical, a firm based in Tarrytown, New York, believes its rheumatoid arthritis drug kevzara would be similarly effective in treating critically ill patients. The drug consists of antibodies that bind to and inactivate the tiny protein molecules on the surface of the body's immune cells known as interleukin 6 that play a role in amplifying an immune response.
"People are dying because they are losing the ability to breathe—because their lungs are filling with inflammation," says George D. Yancopoulos, Regeneron's President and Chief Scientific Officer. "That's what's happening. That's a fact. The question is, what's causing the inflammation? If you shut that off, basically the lungs calm down, the cells leave the lung and they are also not making all this bad stuff."
Regeneron is currently talking with the FDA and the U.S. Department of Health and Human Services about fast-tracking clinical trials. It is enrolling 400 patients hospitalized for COVID-19. If all goes well, it could reach a verdict on the treatment in the next month or two. The company already has produced enough of the medicine to treat tens of thousands of severely affected patients, says Yancopoulos. Sanofi, which has the license to distribute the drug outside the U.S., is initiating similar trials in Europe.

Regeneron is also looking into using novel monoclonal antibodies as a potential weapon against COVID-19. These are custom-made proteins designed by the immune system specifically to bind to and neutralize the virus. Regeneron is using mice that have been genetically engineered to produce antibodies that could be used in the human body. The company has already exposed these "VelociMice" to the COVID-19 virus and extracted thousands of antibodies, and is now screening them for potential effectiveness against COVID-19, and identified a few of the most potent antibodies. It will then mass-produce them by growing them inside cell lines incubated in huge "bioreactors," engineered to promote maximum reproduction.
Christos Kyratsous, Regeneron's vice president for research, says it will take about four months to go from picking the most potent antibodies to producing enough cells to provide the tens of thousands of liters of medicine needed to make the drug widely available to those suffering from COVID-19 in the U.S., leading to hopes by some on the front lines that a new custom-made medication could be in place by the end of August.
Other experimental efforts are aimed at helping patients fight off the infection itself. In mid-March, immunologists and medical professionals at Johns Hopkins University submitted plans to the university's institutional review board and the FDA to extract antibodies from the blood of patients who have already recovered from COVID-19, says Arturo Casadevall, an immunologist and infectious disease expert at Johns Hopkins School of Medicine. The idea is to infuse new patients with antibodies filtered out of the blood of patients who have already successfully fought off the infection.
Doctors facing pandemics have used a similar strategy to combat infectious diseases for more than a century, including the 1918 flu. But this time, the approach has a modern twist. Casadevall and his colleagues plan to rely on methods and equipment that hospitals already have in place in blood banks, such as machines that currently remove antibodies from the blood of patients with autoimmune diseases, to prevent their bodies from attacking their own cells. (The blood is usually reinfused into their bodies to prevent anemia). These same machines could be used instead to extract antibodies from COVID-19 survivors. Scientists would test the antibodies to find the most potent ones and then administer them to sick patients or medical personnel in need of protection. This method could be deployed in cities around the nation, or around the globe—anywhere where blood baking facilities exist. And Casadevall says he has been in contact with health officials at the Mayo Clinic, in New York City and elsewhere, who are considering taking similar measures.
Although the approach would not immediately yield a drug that could be mass produced, it could serve as a stop-gap treatment, he says, until new drugs, like those being developed by Regeneron, come online. "We can put this in place and we can provide people something more than a respirator to provide oxygen," says Casadevall.
A team that included Hopkins infectious disease experts, blood-banking officials and regulatory personnel has been holding regular conference calls. The team is now testing blood samples and developing a plan to deploy the approach throughout Baltimore. He expects that the first filtered antibodies could be fielded by the beginning of April, in time for a "second wave" of patients to hit the hospitals. The approach, already in use in China, could become widespread in the U.S.
The kitchen sink
Doctors on the front lines of the battle in China, Italy and elsewhere have identified other potential treatments by taking a "kitchen sink" approach that uses every available tool to defeat the virus. Because the outbreak is so recent, solid data isn't available on these kinds of measures, but doctors have given favorable anecdotal reports and have administered scores of ad-hoc trials.
The most promising and widely discussed is remdesivir, a broad-spectrum antiviral drug produced by Gilead. Developed originally to treat Ebola patients, remdesivir works by blocking an enzyme that is crucial for the ability of the viruses to reproduce. The drug did not prove effective for Ebola, but trials demonstrated that it did not have serious side effects. Subsequent studies on non-human primates suggest that the drug is effective against coronaviruses—specifically, Middle East Respiratory Syndrome, or MERs—which has given some public health officials cause for optimism.
"There's only one drug right now that we think may have real efficacy, and that's remdesivir," said Bruce Aylward, a senior advisor and international leader of the World Health Organization's joint mission to China, at a Feb. 24 press conference.
Clinical trials to test the drug are already underway in the U.S. and in China's Hubai province. Preliminary results from the first of those studies are expected as soon as April, says Gilead. Gilead is also in the process of enrolling about 1,000 patients, mostly in counties that have already had high numbers of diagnosed cases, in a trial to evaluate the drug given intravenously.
So-called protease inhibitors have also emerged as potential candidates to treat COVID-19. These antiviral drugs, developed during the HIV/AIDS crisis, act on the enzyme protease, which plays a vital role in the ability of HIV to replicate inside the cells that it infects (it chops up big protein molecules into smaller ones). By inhibiting the action of protease, the drugs prevent the progress of an HIV infection, keeping AIDS from developing. Since then, researchers have also developed modified protease inhibitors to fight hepatitis C and other viruses.
Coronaviruses like SARS-CoV-2 also use a type of protease during replication, but the virus is different enough that HIV antivirals may not be effective. Research is ongoing to find out.

The antimalarial drug chloroquine, and its derivative, hydroxychloroquine, are also candidates for COVID-19 treatments. Researchers first began testing their ability to halt the spread of viruses during the battle against AIDS. The drugs are designed to interfere with "endocytosis," the process by which a virus or other microbe enters a cell. They have since been shown to have some success in the lab against a wide range of viral diseases including the common cold and the SARS virus. On March 16, Chinese public health officials announced that a clinical trial at 10 hospitals in Beijing, Guangdong and Hunan Provinces involving more than 100 patients showed a positive effect—patients who took chloroquine were more likely to show a reduction in fever, showed clearer lungs on CT scans and reduced the amount of time to recover.
More treatments will emerge as doctors and scientists on the front lines continue to try new drugs. For instance, in March, a Chinese official said that the drug favipiravir, developed by Fujifilm Toyama Chemical as an influenza drug, showed positive results for COVID-19 patients in trials in Wuhan and Shenzen.
Scaling up
There are many obstacles to getting a treatment out of the lab and into the hospital. First, clinical trials must show that the drugs work safely, and many drugs typically fail this test. A cocktail of the HIV drugs lopinavir and ritonavir, which were being tested in China, was reported to have no benefit to patients. The effectiveness of HIV drugs against COVID-19 remains largely anecdotal and unproven. And choloquine in high doses can prove toxic.
Once a drug is proved safe and effective, getting it to millions of patients around the world requires a massive manufacturing capacity. Ramping up can take months, says Prashant Yadav, a visiting fellow at the Center for Global Development and an expert on healthcare supply chains. For instance, he estimates it would likely take six months to a year to sufficiently ramp up production to meet the potential global demand for remdesivir, should it prove effective and safe.
Given the urgent need for new drugs around the world, some public health officials have called for new protocols to determine who will decide how to allocate limited supply. There would have to be a way of coordinating the supply of drugs, with clear roles and responsibilities for fast-tracking treatments. This would involve an unprecedented level of coordination among the World Health Organization, organizations that finance global health measures, supply-chain experts in the pharmaceutical companies and governments. Once a country has obtained a drug, the government together with private health care organizations and drug companies have to fast-track distribution of the drugs.
"Can governments and global agencies make extremely fast decisions in the complex and somewhat uncertain environment?" asks Yadav. "How do we run a supply system so that every hospital that orders it can get sufficient supply? It's a capacity rationing problem: someone has to decide how much of demand will we need for existing supply. And as we know, rationing decisions bring out the worst in terms of global coordination and local and national politics. And if a company has never sold much in Africa then they will have to start from scratch."
Long-term fix
Anti-viral treatments can hopefully keep people from dying from COVID-19, but the best long-term hope to control the disease is a vaccine. The typical timeline for vaccine development is 12 to 18 months. The most promising and advanced is mRNA1273, which is being developed by Moderna, a Boston company. In mid-March, Kaiser Permanente Washington Health Research Institute began a safety and dosing trial in which 45 young, healthy volunteers will receive different doses of the vaccine.
Other efforts include INO-4800, a vaccine being developed by Pennsylvania-based Inovio Pharmaceuticals; a vaccine based on previous work against the Avian coronavirus from MIGAL Research Institute in Israel; a company called Heat Biologics, which already has a cancer vaccine in clinical trials, as well as efforts in early stages from Johnson and Johnson, Pfizer and GSK.
Few doubt that at least some of these efforts, and many others like them, will eventually result in effective treatments. How long that will take depends on a lot of hard work and some luck. "Against all odds, we figured out to mass produce penicillin, we beat polio and smallpox," Dr. Peter Jay Hotez professor and dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston. "It's unfortunate we have to wait until things got so dire to focus on the needs of the world, but I think we are there now."
Correction, 3/20/2020, 10:34 am: This story was changed to reflect the fact that Regeneron trials for Kevzara are starting with 400 patients, not a few as previously reported.
Correction, 3/20/2020, 1:35 pm: The drug chloroquine works by interfering with endocytosis, the process by which a virus enters a cell, not exocytosis as previously reported; the story has been modified to correct the error.
Correction, 3/20/2020, 2:32 pm: The COVID-19 virus has 30,000 nucleotides of RNA, not 3 billion of DNA as previously reported; this has been corrected in the story.














